2,4,6-Trinitrobenzenesulfonic acid (TNBS) Induced Colitis in Mice (and Rat)

Back to Pharmacology and Toxicology Assessment top page
Induction:
On study day 0, mice are infused via rectal catheter with 2,4,6-Trinitrobenzenesulfonic acid (TNBS) (50 µL 50% EtOH) to induce colitis. Mice are randomized into groups and are fasted for 12 to 16 hours prior to TNBS administration.
Disease Parameters:
The TNBS murine model of colitis is a Th1 inflammation characterized by infiltration of CD4+ lymphocytes that shares features with human Crohn’s disease (CD) and ulcerative colitis (UC)1, 2. In this animal model, male SJL mice are challenged via rectal catheter with TNBS to induce inflammation in the colon characterized by intense hyperemia, edema, and gut wall thickening3.
Dosing Paradigms:
- Dosing is initiated prior to or on the day of TNBS administration (Day 0), and is continued until study termination on Day 4.
- Route of administration: SC, PO, IP, IV, IC
Clinical Assessment:
Mice are weighed on study days 0 through 4. Disease activity is scored on days 0 through 4 using the following criteria:
Weight Loss (%):
0 = ≤ 2%
1 = 3 to 6%
2 = 7 to 12%
3 = > 12%
Stool Consistency:
0 = Normal Stool (well formed pellets)
1 = Few Formed Pellets to Semi-solid Stool
2 = Diarrhea (liquid stool that adheres to the anus)
3 = No Stool
Occult/Gross Blood (Using Hemoccult Test):
0 = Normal (no blood in stool)
1 = Blood in Stool (formed pellets)
2 = Bloody Fluid (diarrhea)
3 = Anogenital Staining (no stool)
On study day 4, animals are necropsied. Colons are removed, measured for length, weighed, and assessed for gross morphologic changes. Colon contents are scored at necropsy according to the following criteria:
0 = normal, no blood observed
1 = minimal/mild damage, hint of blood, soft stool
2 = moderate/marked damage, blood tinged, soft to liquid stool
3 = severe damage, bloody content, liquid stool or no stool
Histopathological Assessment:
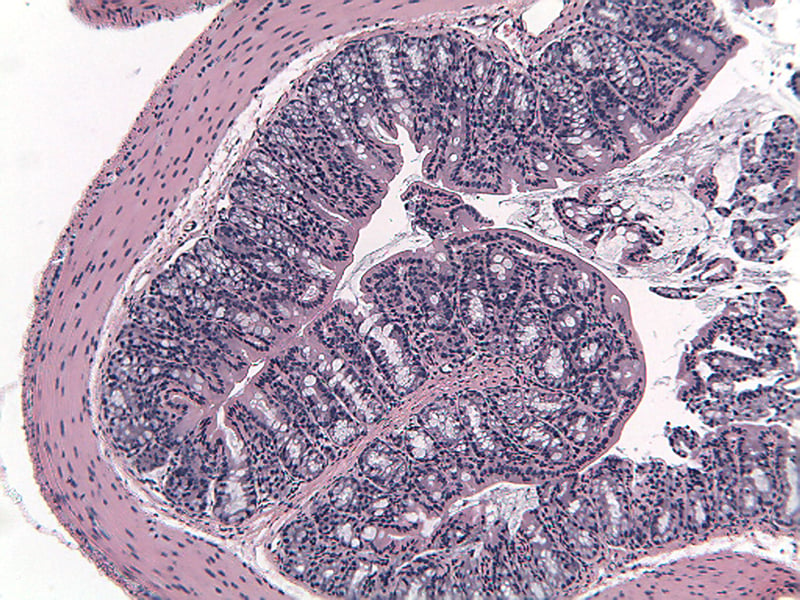
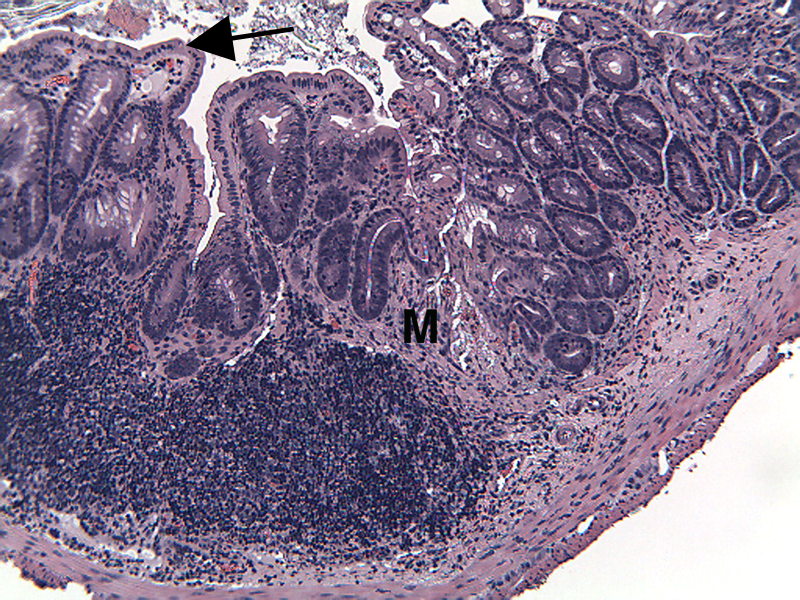
Colons are collected for processing and embedding, and sections are stained to quantitate inflammation, gland loss and epithelial loss, scored according to to these methods.
Sample Data (Click on image to enlarge):
Representative Photomicrographs of Colons
For additional examples of positive controls, please contact us.
Notes:
TNBS colitis exhibits heightened Th1-Th17 response (increased IL-12 and IL-17) as the disease becomes chronic, and IL-17 signaling may represent a target for therapeutic intervention2, 4. Treatments that have demonstrated efficacy in this model include P13Kγ inhibitors, helminth soluble proteins, and activation of the aryl hydrocarbon receptor (AhR)5, 6, 7.
Optional Endpoint
- PK/PD blood collections
- Cytokine/chemokine analysis via Luminex(R)
- Other sandwich ELISAs
- CBC/clinical chemistry analysis
- Soft tissue collection
- Histopathologic analysis
- Immunohistochemistry analysis
- Endoscopy
References
- Elliott DE, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 284:G385–G391, 2003.
- Zhang Z, Zheng M, Bindas J, et al. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 12(5):382–388, 2006.
- Selve N and Wohrmann T. Intestinal inflammation in TNBS sensitized rats as a model of chronic inflammatory bowel disease. Mediators Inflamm. 1:121–126, 1992.
- Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 15(3):341–352, 2009.
- Dutra RC, Cola M, Leite DFP, et al. Inhibitor of P13Kg ameliorates TNBS-induced colitis in mice by affecting the functional activity of CD4+CD25+FoxP3+ regulatory T cells. Br J Pharmacol. 163:358–374, 2011.
- Ruyssers NE, DeWinter BY, DeMan JG, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 15(4):490–500, 2009.
- Benson JM and Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 120(1):68–78, 2010.
Related Pages
- MDR1a Spontaneous Colitis In Mice
- Adoptive T-Cell Transfer In Mice
- Anti-CD40 Colitis In Mice
- Dextran Sulfate Induced Colitis (DSS) In Mice
- Dextran Sulfate Salt Induced Colitis (DSS) In Rat
- Indomethacin Induced Crohn’s In Rats
- 2,4,6-Trinitrobenzenesulfonic Acid (TNBS) Induced Colitis In Mice